Abstract
Objectives
Hips with metal-on-metal total hip arthroplasty (MoM THA) have a high rate of adverse local tissue reactions (ALTR), often associated with hypersensitivity reactions. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) measures tissue perfusion with the parameter Ktrans (volume transfer constant of contrast agent). Our purpose was 1) to evaluate the feasibility of DCE-MRI in patients with THA and 2) to compare DCE-MRI in patients with MoM bearings with metal-on-polyethylene (MoP) bearings, hypothesising that the perfusion index Ktrans in hips with MoM THA is higher than in hips with MoP THA.
Methods
In this pilot study, 16 patients with primary THA were recruited (eight MoM, eight MoP). DCE-MRI of the hip was performed at 1.5 Tesla (T). For each patient, Ktrans was computed voxel-by-voxel in all tissue lateral to the bladder. The mean Ktrans for all voxels was then calculated. These values were compared with respect to implant type and gender, and further correlated with clinical parameters.
Results
There was no significant difference between the two bearing types with both genders combined. However, dividing patients by THA bearing and gender, women with MoM bearings had the highest Ktrans values, exceeding those of women with MoP bearings (0.067 min−1versus 0.053 min−1; p-value < 0.05) and men with MoM bearings (0.067 min−1versus 0.034 min−1; p-value < 0.001). Considering only the men, patients with MoM bearings had lower Ktrans than those with MoP bearings (0.034 min−1versus 0.046 min−1; p < 0.05).
Conclusion
DCE-MRI is feasible to perform in tissues surrounding THA. Females with MoM THA show high Ktrans values in DCE-MRI, suggesting altered tissue perfusion kinematics which may reflect relatively greater inflammation.
Cite this article: Dr P. E. Beaule. Perfusion MRI in hips with metal-on-metal and metal-on-polyethylene total hip arthroplasty: A pilot stud. Bone Joint Res 2016;5:73–79. DOI: 10.1302/2046-3758.53.2000572.
Article focus
-
We explored the technical feasibility of DCE-MRI near total hip arthroplasty (THA) hardware.
-
We hypothesised that the perfusion index Ktrans is higher for metal-on-metal (MoM) implants than for metal-on-polyethylene implants.
Key messages
-
DCE-MRI near THA hardware is feasible using fast spin echo pulse sequences.
-
The highest Ktrans values were found in women with MoM implants.
Strengths and limitations
-
This is the first time DCE-MRI has been performed near THA hardware in humans.
-
It was not possible to measure the arterial input function.
-
Small patient numbers limit the interpretation of data.
Introduction
Total hip arthroplasty (THA) with metal-on-metal (MoM) bearings was reintroduced in the late 1980s. At that time, MoM THA was hypothesised to minimise wear and convey longer survivorship than standard metal-on-polyethylene (MoP) THA. Although small diameter femoral head MoM THAs and certain designs of hip resurfacing have indeed done well in large clinical series and registries, large diameter femoral head (> 36 mm) MoM THAs have had high rates of adverse local tissue reactions (ALTR) and revisions.1-4 Unfortunately, it is not clear which of these hundreds of thousands of patients’ THAs are at risk to fail.
Ideally, these ALTRs should be diagnosed prior to significant soft-tissue damage developing. Recent pathological analyses of these ALTRs, as well as associated pseudotumours, reveal that the underlying physiology is of a hypersensitivity reaction associated with regional hypervascularity.5 At this time there are no validated, definitive prognostic tests that can predict ALTR. Most commonly, the investigation includes radiological imaging (MRI), computed tomography (CT), ultrasound (US) and metal ion level testing at the time of clinical presentation. However, the radiological findings may be non-specific and the findings may not correlate with clinical severity or significance.3,6 Because of the associated hypervascular response with ALTR, a potential prognostic test is dynamic contrast-enhanced MRI (DCE-MRI). This technique involves rapid, repeated imaging before, during and after intravenous injection of a gadolinium-based contrast agent7 providing a quantitative index Ktrans, a parameter that reflects blood flow and perfusion in tissue. More importantly, Ktrans has proven useful for assessing vasculature and inflammation, as well as predicting the future course of some microvessel-dependent diseases.8-11 To our knowledge, Ktrans measurements have never been performed near human THA. The purpose of this study was, 1) to evaluate the feasibility of Ktrans measurements of the peri-articular tissue bed in patients with a THA and 2) to compare Ktrans in patients with a MoM bearing versus MoP, hypothesising that Ktrans in hips with MoM THA is higher than in hips with MoP.
Materials and Methods
The recruitment for this study took place between July 2007 and May 2010 at the Ottawa Hospital. Ethical approval was obtained from the Ottawa Hospital Research Ethics Board, and the trial was registered on www.clinicaltrials.gov with registration number NCT00911599. The study was designed as level three evidence as subjects were originally recruited as part of a prospective randomised study quantifying acetabular bone mineral density comparing a monoblock cobalt-chrome large head MoM bearing with a standard MoP bearing in primary THA.12 This study protocol included the evaluation of serum metal ion levels. Inclusion criteria were any patient between 45 and 75 years of age who had undergone primary THA for non-inflammatory degenerative joint disease, including osteoarthritis (degenerative or post-traumatic), congenital hip dysplasia and avascular necrosis. Exclusion criteria included previous arthrodesis of the hip, Girdlestone procedure, acute fracture of the femoral neck, above-knee amputations, significant knee arthritis (including previous total knee arthroplasty), evidence of active infection, neurological or muscular disease that may adversely affect gait or weight-bearing, previous ipsilateral hemi-/total resurfacing, uni/bipolar arthroplasty or THA. Patients with neuropathic joints, a requirement for structural bone grafts, documented severe psychiatric disease or a documented allergy to cobalt chromium molybdenum were also excluded from participation.
At the time of this study, 36 of the 50 randomised patients had minimum two-year follow-up and of this group, two were deceased, one had renal failure, one had a fracture and two had undergone a revision, leaving 30 patients eligible for the study. All of these patients were contacted by mail and 24 patients agreed to participate. Three of these patients were unreachable to book the MRI and three patients changed their minds citing claustrophobia, leaving 18 patients that had imaging completed. Due to poor image quality, two of the patients who had the MRI were excluded. The poor images were likely to be due to excessive movement during the image acquisition.
From this cohort, 16 asymptomatic patients were recruited with a mean age at MRI of 65 years (standard deviation (sd) 6) (55 to 74). Seven (44%) of the patients were males. Of the seven male patients, five had a MoM THA and two a MoP THA. Of the nine females, three had a MoM THA and six a MoP. The body mass index (BMI) of the patients was 28.4 kg/m2 (sd 4.7) (23 to 38). The MRIs were performed between October 2011 and August 2012, on average 3.6 years (sd 0.7) (2.4 to 4.7) after arthroplasty implantation. There were eight patients in each group of large head MoM THA (BFH, MicroPort, Memphis, Tennessee) and MoP THA (Lineage shell, MicroPort). The median femoral head sizes were 48 mm (40 to 50) and 28 mm (28 to 36) for MoM and MoP, respectively. Prior to surgery, all patients had filled out a questionnaire with the Western Ontario and McMaster Universities Arthritis index (WOMAC) and the University of California at Los Angeles (UCLA) activity score. All patients repeated the questionnaires two years after surgery. In addition, serum cobalt and chromium levels had been taken at the two-year mark. Peripheral blood was collected, by venipuncture of the median cubital vein, into 6 mLtrace-element evacuated plastic tubes with clot activator (BD Diagnostics, Franklin Lakes, New Jersey). The blood was mixed immediately with the additive by gently inverting the tubes five times, allowed to clot for 30 minutes at room temperature, and centrifuged for ten minutes at 1600 × g at 4°C. The serum was transferred into 8 mL polypropylene screw-cap tubes (Sarstedt Inc., Saint-Léonard, Quebec) using a polyethylene plastic transfer pipette (VWR, Mississauga, Ontario), and frozen/stored at -20°C. Concentrations of Co and Cr were measured by inductively coupled plasma mass spectrometry (London Laboratory Services Group, London Health Sciences Centre, London, Ontario, Canada). Corrections for Co and Cr contamination originating from the containers were not required.
MRI was performed on a 1.5 Tesla (T) scanner (Magnetom Symphony; Siemens AG, Medical Solutions, Erlangen, Germany). Before DCE-MRI, standard anatomic imaging was performed with T1-weighted fast spin echo (FSE), and short tau inversion recovery (STIR) sequences in the axial, coronal and sagittal planes. Metal artefact reduction strategies were incorporated into the protocol, including increased bandwidth, use of fast spin echo as opposed to spin echo or gradient recalled echo sequences, minimisation of the echo time, and minimising voxel size by increasing the frequency encode direction steps. In addition, the number of averages and phase oversampling was increased in order to recover some of the signal that may be lost by the artefact minimisation protocol. The parameters for T1-weighted FSE were bandwidth = 385 Hz/pixel, TR = 649 ms, TE = 9.1 ms, acquisition matrix = 448 x 224, number of averages = 2. The parameters for STIR were bandwidth = 395 Hz/pixel, TR = 4500 ms, TE = 34 ms, acquisition matrix = 384 x 211, number of averages = 2, and inversion time = 155 ms.
DCE-MRI was performed using the “Bookend” method developed in 1999 by Cron, Santyr and Kelcz.13 Rapid, repeated, T1-weighted imaging with injection of contrast agent was flanked in time by two “Bookend” spin-lattice relaxation time (T1) inversion recovery measurements. In other words, a T1 measurement was performed, followed by dynamic imaging with contrast, followed by a second T1 measurement. The two T1 measurements served to convert the dynamic signal-versus-time to gadolinium concentration- versus -time.13 FSE was used for all pulse sequences of the Bookend method, in order to minimise metal artefacts. FSE parameters were: echo train length = 7, bandwidth = 349 Hz/pixel, echo time = 6.8 ms, number of slices = 16, slice thickness = 6 mm, gap between slices = 1.5 mm, field of view = 22 cm, and matrix size = 128 x 128. The images were acquired in the axial plane with slices prescribed between the level of the lesser trochanter and the acetabular roof. Each T1 measurement was performed with repetition time = 8000 ms and an inversion pulse with inversion times = 500, 1000, and 3000 ms. T1 was computed for each voxel by fitting an exponential recovery curve. The dynamic imaging with contrast was performed with repetition time = 865 ms, no inversion pulse, temporal resolution = 7.8 s, gadolinium contrast agent dose = 0.1 mmol/kg (Magnevist; Bayer, Berlin, Germany), injection rate = 2.5 cc/sec, and duration of DCE-MRI = 4 min. The total scan time of the Bookend method was 11 minutes.
For each voxel, a gadolinium concentration-versus-time curve was estimated from the two T1 measurements and the dynamic signal-versus-time curve was estimated using the Bookend method.13 Contrast agent accumulation (in millimolar) was calculated for each voxel using the end of the concentration-versus-time curve. The concentration-versus-time data were then analysed voxel-by-voxel with nordicICE software (NordicNeuroLab, Bergen, Norway) using the Extended Tofts Model to generate three-dimensional maps of Ktrans.14 For this modeling, a population-based arterial input function was used.15 Example DCE-MRI data are shown in Figures 1 and 2.
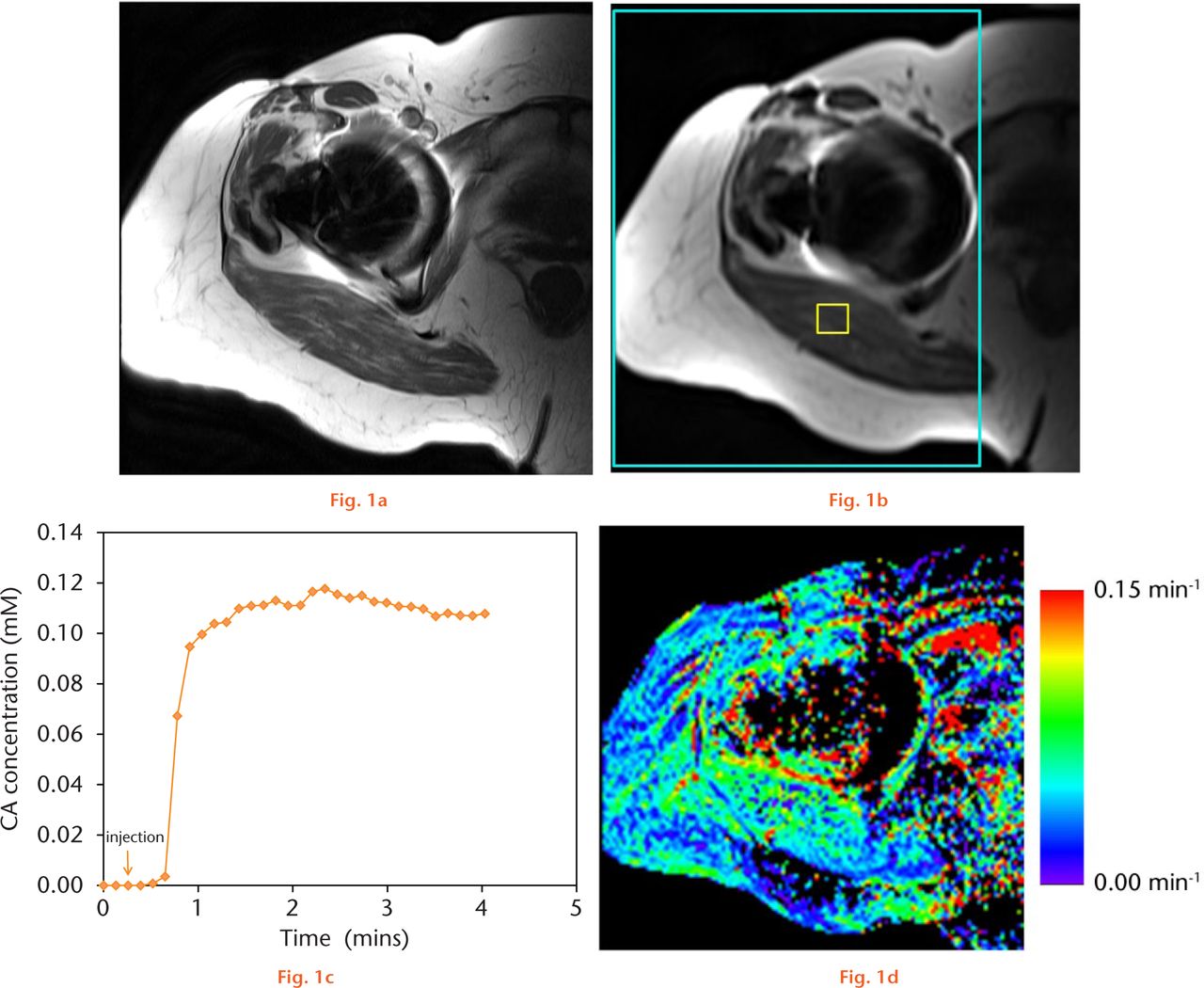
Fig.
Example dynamic contrast enhanced (DCE)-MRI dataset for one patient. a) One slice of a high-resolution T1-weighted FSE sequence (standard-of-care anatomic imaging performed before DCE-MRI). b) Same slice location with one image of the DCE-MRI sequence. Small yellow box shows an region of interest (ROI) in muscle. Large cyan-coloured box shows the whole-tissue ROI used for mean Ktrans calculations. c) Example concentration-vs-time curve in the muscle ROI. d) Resultant Ktrans map, in units of min−1. The mean Ktrans value (counting non-zero values only), standard deviation, and standard error for this patient were 0.062, 0.049, and 0.00015, respectively.
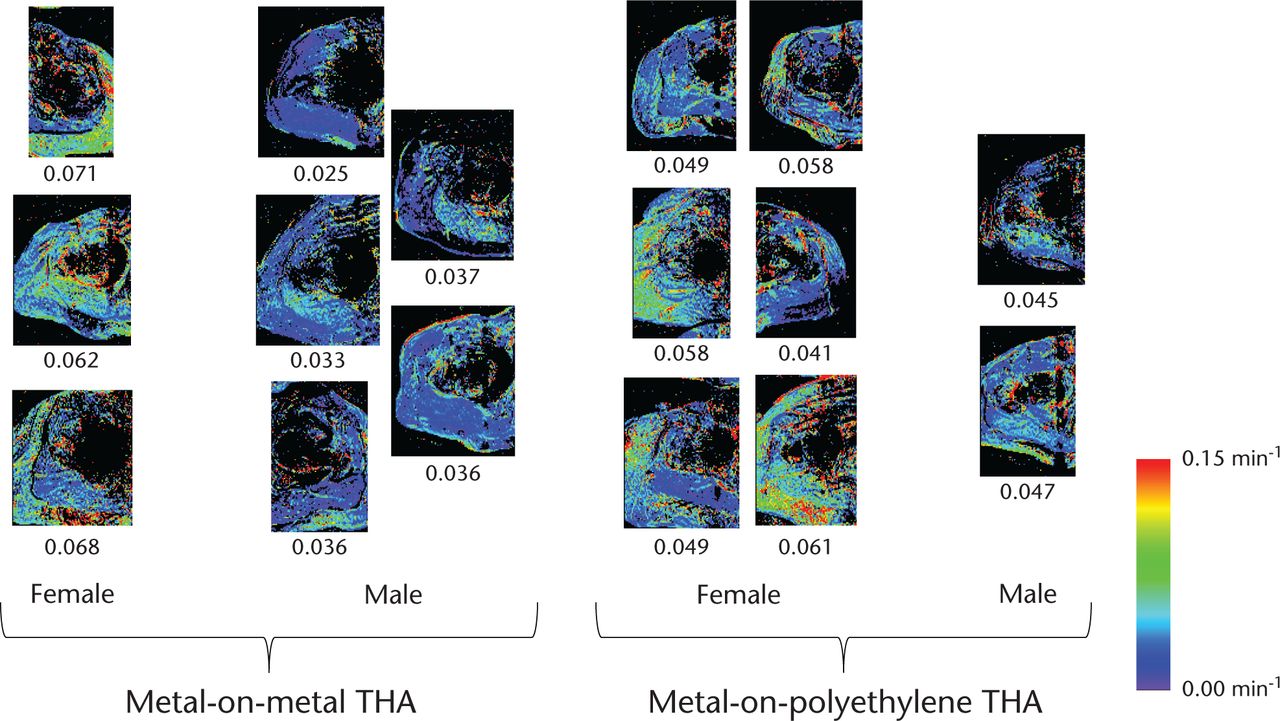
Fig. 2
All Ktrans maps (one slice shown per patient), grouped by THA implant type and gender. The maps have been cropped to show only the regions lateral to the bladder which were used for computations. Mean Ktrans value (in units of min−1) is displayed under each map. For each patient, the standard deviation across all Ktrans values was approximately 0.8 times the mean Ktrans value. The standard error was approximately 0.002 times the mean Ktrans value.
A single, mean Ktrans value for the entire peri-prosthetic tissue bed (including peri-prosthetic soft tissue, muscle and fat) lateral to the bladder was calculated for each patient. Image review was performed by a fellowship-trained musculoskeletal radiologist (KR) with nine years of experience. The radiologist drew a rectangular region of interest (ROI) on each of the 16 DCE-MRI axial slices. The height (anterior-posterior direction) of the ROI spanned the entire image (22 cm). The position and width of the ROI in the left-right direction were chosen to include all tissue in the image lateral to the bladder (including peri-prosthetic soft tissue, muscle and fat), excluding the bladder and any tissue anterior or posterior to it. The mean Ktrans value (excluding zero values, characteristic of those voxels highly affected by the metal artefact) for all voxels in all slices within these ROIs was then computed. Considering only muscle, contrast agent accumulation was estimated to be 0.10 ± 0.02 mM, with Ktrans values 0.04 ± 0.01 min−1, in line with the literature.16,17
SPSS software (IBM Corp, Armonk, New York.) was used for statistical analysis. The Independent samples t-test (student t-test) was used to compare parameters between hips with MoM and MoP bearings. Normal distribution was tested using the Kolmogorov-Smirnov test. Correlation coefficients between Ktrans values and clinical parameters including metal ion concentration in the serum, BMI, age, WOMAC and UCLA (pre- and post-operative) were calculated using the Pearson test. We assumed statistical significance at p-value < 0.05. Correlation coefficient of 0.20 to 0.39 was defined as a weak, 0.40 to 0.59 as a moderate, 0.6 to 0.79 as a strong, and ≥ 0.8 as a very strong correlation.
Results
Considering all Ktrans data (all tissues, both genders combined), there was no difference between the two bearing types (mean Ktrans value for MoM hips = 0.046 min−1versus 0.051 min−1 for MoP hips; p > 0.05) (Table I). A significant correlation between Ktrans values and titanium concentration in the serum was observed (R = 0.760, p = 0.011), but not with chromium and cobalt serum levels (p > 0.05). Chromium and cobalt serum levels were significantly higher in patients with MoM THA than in patients with MoP THA (p < 0.01), but titanium levels were not. Correlation of Ktrans versus BMI, age, WOMAC or UCLA (pre- or post-operative) was not significant (p > 0.05), considering all patients combined or by gender subanalysis.
Table I.
Demographics, clinical scores, blood analyses and Ktrans values in subjects with metal-on-metal (MoM) bearing compared with metal-on-polyethylene (MoP) bearing, divided by gender
| Parameter |
MoM |
MoP |
||||
|---|---|---|---|---|---|---|
| Hips (patients) | Total8 (8) | Men5 (5) | Women3 (3) | Total8 (8) | Men2 (2) | Women6 (6) |
| Ktrans (min−1) | 0.046 (0.018) (0.025 to 0.071) | 0.034 (0.005) (0.025 to 0.037)† | 0.067 (0.005) (0.062 to 0.071) | 0.051 (0.007) (0.041 to 0.061) | 0.046 (0.002) (0.040 to 0.047) | 0.053 (0.008) (0.041 to 0.061) |
| Age (yrs) | 64.5 (6.2) (55 to 72) | 65.6 (6.3) (55 to 72) | 62.7 (6.8) (55 to 68) | 65.3 (5.3) (56 to 74) | 63.0 (1.4) (62 to 64) | 66.0 (6.0) (56 to 74) |
| BMI (kg/m2) | 29.1 (4.2) (25.0 to 34.4) | 29.7 (5.3) (25.0 to 34.4) | 28.3 (3.0) (25.8 to 31.6) | 27.7 (5.8) (23.3 to 37.9) | 24.6 (1.3) (23.7 to 25.5) | 29.0 (6.6) (23.3 to 37.9) |
| Baseline WOMAC | 54.7 (23.9) (11.3 to 87.9) | 55.4 (15.5) (37.3 to 78.0) | 53.6 (38.9) (11.3 to 87.9) | 49.2 (14.6) (26.3 to 70.3) | 40.6 (0.6) (40.2 to 41.0) | 52.1 (16.1) (26.3 to 70.3) |
| Baseline UCLA | 3.7 (2.0) (2 to 7) | 3.0 (1.4) (2 to 5) | 4.7 (2.5) (2 to 7) | 4.6 (2.5) (3 to 6) | 4.5 (2.1) (3 to 6) | 4.7 (1.5) (3 to 6) |
| Follow-up WOMAC | 91.8 (9.5) (78.1 to 100) | 88.2 (10.5) (78.1 to 100) | 97.9 (2.8) (94.8 to 100) | 86.0 (13.9) (60.0 to 100) | 89.1 (0.7) (88.5 to 89.6) | 85.0 (16.3) (60.0 to 100) |
| Follow-up UCLA | 7.1 (0.8) (6 to 8) | 7.2 (0.8) (6 to 8) | 7.0 (1.0) (6 to 8) | 6.6 (1.2) (5 to 8) | 7.5 (0.7) (6 to 8) | 6.3 (1.2) (5 to 8) |
| Titanium serum concentration (mg/ml) | 2.9 (3.1) (1.1 to 7.4) | 1.4 (0.4) (1.1 to 1.8)† | 7.4 | 2.7 (0.6) (1.7 to 3.4) | 2.95 (0.32) (2.73 to 3.18) | 2.55 (0.69) (1.76 to 3.40) |
| Chromium serum concentration (mg/ml) | 1.9 (1.2) (1.0 to 4.4)* | 2.0 (1.6) (1.0 to 4.4) | 1.7 (0.8) (1.0 to 2.5) | 0.4 (0.4) (0.1 to 1.0) | 0.24 (0.06) (0.19 to 0.28) | 0.44 (0.45) (0.06 to 1.21) |
| Cobalt serum concentration (mg/ml) | 3.6 (2.6) (0.8 to 8.4)* | 4.5 (2.9) (1.4 to 8.4) | 2.3 (1.9) (0.8 to 4.4) | 0.3 (0.1) (0.2 to 0.5) | 0.29 (0.11) (0.22 to 0.37) | 0.27 (0.12) (0.17 to 0.47) |
-
*
Significant difference between MoM and MoP bearing
-
†
Significant difference between gender with MoM bearing
-
Values of continuous parameters are expressed as mean and standard deviation with range in parentheses; BMI, body mass index; WOMAC, Western Ontario McMasters Universities Osteoarthritis index; UCLA, University of California, Los Angeles
Gender subanalysis did, however, reveal that males with MoM bearings had significantly lower Ktrans values than those with MoP bearings (0.034 min−1versus 0.046 min−1; p = 0.019) whereas females with MoM bearings had significantly higher Ktrans values than those with MoP bearings (0.067 min−1versus 0.053 min−1; p = 0.024) and higher than males with MoM bearings (0.067 min−1versus 0.034 min−1; p = 0.000) (Fig. 3). There was no significant difference in serum concentration of chromium and cobalt and in clinical scores in MoM THA between men and women (p > 0.05). None of the patients was diagnosed with ALTR and none underwent revision within two years of surgery.
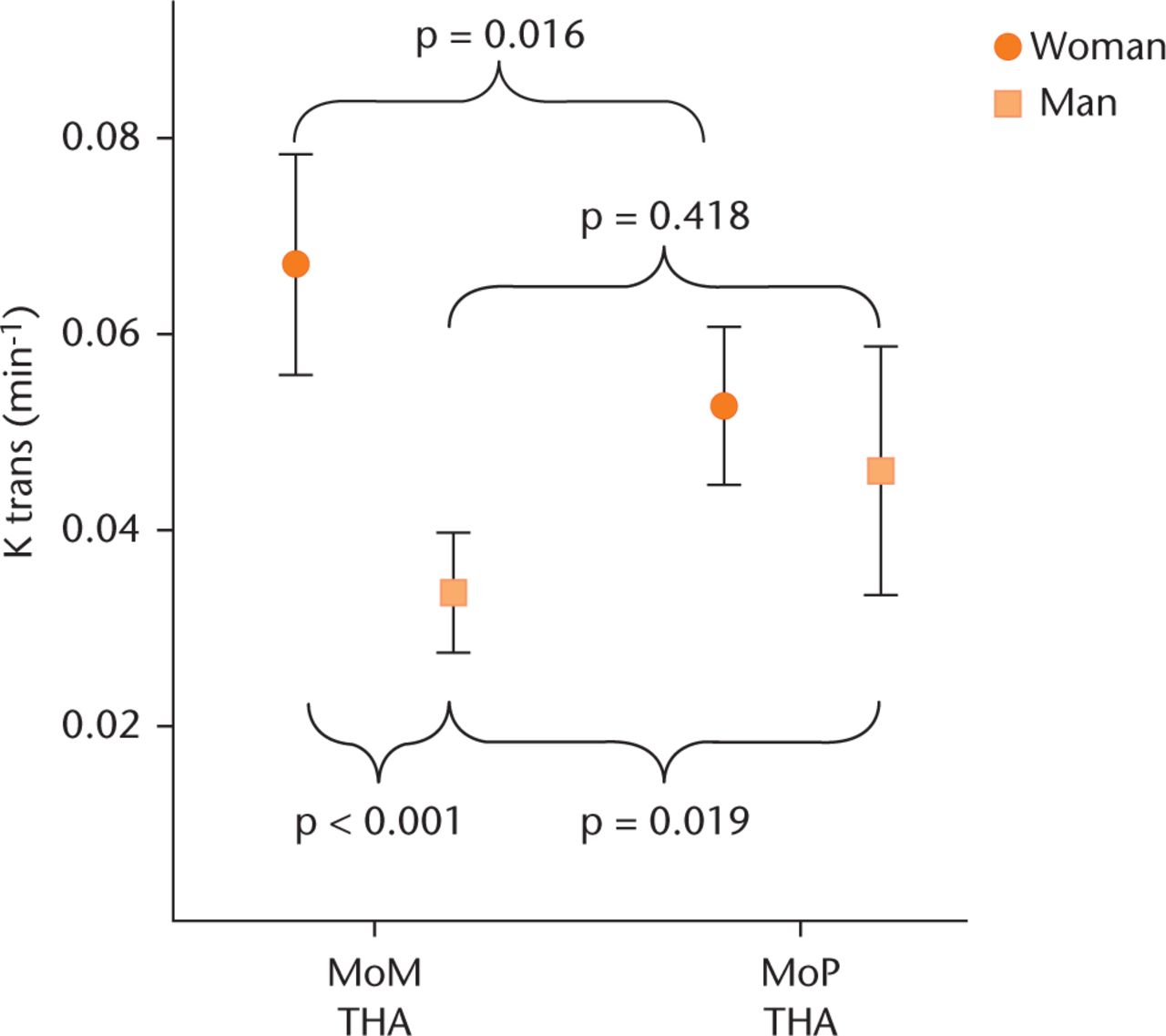
Fig. 3
Ktrans values in hips with a total hip arthroplasty, divided by bearing and gender. Error bars show 95% confidence intervals. Ktrans values are significantly increased in females with metal-on-metal (MoM) bearing compared with males with MoM bearing and females with metal-on-polyethylene (MoP) bearing. Males with MoM bearing show significantly lower Ktrans values than males with MoP bearing.
Discussion
Understanding the pathological mechanism of implant failure remains a challenge. Several factors may influence this failure, including implant positioning, implant material, patient activity and the biology of the host.18 More importantly, the fact that some patients have failure of their joint arthroplasties with no measurable, or minimal, wear while others with significant wear have minimal to no host response would indicate that the host response to implant wear debris is key variable.19 Consequently, gaining a better understanding of the pathological mechanisms that may influence the development of an inflammatory reaction in the presence of a THA is critical in the development of screening and diagnostic tools for ALTR. When looking at failed MoM THA, Willert et al20 described an aseptic, lymphocyte-dominated, vasculitis-associated lesion (ALVAL), indicating a delayed hypersensitivity reaction. It has also been shown that THA has a shorter lifespan in patients whose skin tests positive for sensitisation to implant components.21 To our knowledge, this is the first report of DCE-MRI data in patients with a THA. Our pilot study has shown that MRI is achievable using a fast spin echo sequence for the DCE-MRI instead of the conventional gradient echo sequence, which would be prone to significant metal artefact.22-24
Different imaging techniques have been applied to evaluate for ALTR, but none of these techniques can differentiate between incidental, asymptomatic changes and symptomatic, more clinically significant changes that may be precursors to joint failure. Conventional radiographs are insensitive for diagnosing ALTR.25 By applying a metal artefact reduction sequence (MARS), MRI of diagnostic quality is feasible in hips with THA. The typical finding in hips with ALTR is a complex peri-prosthetic mass or collection, often with heterogeneous fluid signal.26 This collection often communicates with the hip joint but can extend into the surrounding muscles, intermuscular planes, tendon sheaths and subcutaneous fat.25,26 A grading system for MRI findings has been introduced, but fails to differentiate between benign and aggressive lesions or to determine risk of progression.27 On ultrasound, ALTR has been described as a solid or cystic mass or a complex fluid collection.28
In this study we found that female patients with a MoM bearing have a significantly higher level of perfusion, as measured with DCE-MRI, compared with the MoP group. When looking at the clinical outcome of female patients with MoM THA, pseudotumours, metal-bearing complications, aseptic loosening, and prosthesis revision are significantly higher compared with male patients.29,30 It has been postulated that these increased problems in women are due to wider range of hip joint motion and subsequently more frequent impingement, or higher incidence of metal allergy caused by wearing jewellery.30 Although there is most likely a multifactorial aetiology to these poor outcomes (i.e. fibrous fixation of the implant, soft-tissue irritation, reaction to wear debris), female gender certainly stands out. Looking at patients with MoM THA in this study, women had Ktrans values which were nearly double those of men, possibly indicating a different host response to a MoM bearing. All THAs, regardless of type, may have some level of inflammatory response or perhaps mild alteration in perfusion kinetics of soft tissues. There are two variables that determine the degree of response: gender and THA type. It is therefore possible that the inflammatory profile, from lowest to greatest, is ranked 1) Male MoM 2) MoP (equal for both genders) 3) Female MoM. The pathway for developing soft-tissue reactions is not fully elucidated, and is likely a function of variable implant inflammatory profiles as well as gender-specific physiology. Indeed, gender differences have been reported for cerebral and cardiac perfusion measured with MRI, with 13% to 20% higher values in women, similar to what we found in MoP THA patients (11% higher Ktrans values in women).31,32
With the numbers available, we were unable to identify a correlation between Ktrans and WOMAC scores, UCLA scores, cobalt ion levels, or chromium ion levels. This may not be surprising given that ALTR has not been found to correlate with pain or metal ion serum levels.3,26,33-35 Although Ktrans did not correlate with cobalt and chromium levels, it did correlate with titanium levels (R = 0.760, p = 0.011). This suggests that titanium may have a greater inflammatory profile, or may be of greater relevance than the other ions in the study of ion-related soft-tissue reactions.
An important limitation of this study was the small number of patients in each group. The patients were randomised, in an effort to minimise selection bias. This work was constructed as a pilot study, however, and the fact that significant differences were found despite the small number demonstrates the exciting potential of this imaging technique in diagnosing ALTR. It is unclear if any of these patients will eventually go on to have failure of their joint arthroplasty and more long-term follow-up of this patient cohort is needed. The absent difference of Ktrans values between hips with MoM and hips with MoP bearing cannot be proved with this sample size, as a type II error cannot be excluded.
DCE-MRI images suffered from metal artefacts immediately adjacent to the implant, although this did not significantly affect image quality in the surrounding tissues (Fig. 1). Another limitation was that we were unable to obtain individual arterial input functions. This is because spin echo sequences are particularly sensitive to inflow effects, making it difficult to obtain reliable DCE-MRI curves in blood.36 Only one hip was scanned per patient. It was not feasible to scan both simultaneously as this would have increased the field of view and required a significant increase in scan time or a reduction in resolution. However, doing a separate scan of the contralateral side, or native hip without THA, would have provided an internal reference for each subject, and will be considered for future projects beyond this pilot study.
This study has shown the feasibility of a novel DCE-MRI technique for evaluation of tissue surrounding THA. Higher Ktrans values measured with DCE-MRI were found in female patients with MoM THA, indicating high tissue perfusion. This may reflect gender differences or underlying adverse tissue inflammatory reaction, which may be a precursor to tissue damage. Our findings have potential future clinical and research applications for identifying and stratifying patients of particular gender and/or with various THA types who may be at increased risk for joint failure due to tissue hypersensitivity reactions.
Funding Statement
None declared.
ICMJE conflict of interest
None declared.
References
1. Milošev I , KovaS, TrebšeR, LevašiV, PišotV. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg [Am]2012;94-A:1756-1763. Google Scholar
2. Smith AJ , DieppeP, HowardPW, BlomAW, National Joint Registry for England and Wales. Failure rates of metal-on-metal hip resurfacings: analysis of data from the National Joint Registry for England and Wales. Lancet2012;380:1759-1766.CrossrefPubMed Google Scholar
3. Hart AJ , SatchithanandaK, LiddleAD, et al.. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg [Am]2012;94-A:317-325.CrossrefPubMed Google Scholar
4. Fehring TK , OdumS, SproulR, WeathersbeeJ. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res2014;472:517-522.CrossrefPubMed Google Scholar
5. Campbell P , EbramzadehE, NelsonS, et al.. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res2010;468:2321-2327.CrossrefPubMed Google Scholar
6. Almousa SA , GreidanusNV, MasriBA, DuncanCP, GarbuzDS. The natural history of inflammatory pseudotumors in asymptomatic patients after metal-on-metal hip arthroplasty. Clin Orthop Relat Res2013;471:3814-3821.CrossrefPubMed Google Scholar
7. Gordon Y , PartoviS, Müller-EschnerM, et al.. Dynamic contrast-enhanced magnetic resonance imaging: fundamentals and application to the evaluation of the peripheral perfusion. Cardiovasc Diagn Ther2014;4:147-164.CrossrefPubMed Google Scholar
8. Cousin S , TaiebS, PenelN. A paradigm shift in tumour response evaluation of targeted therapy: the assessment of novel drugs in exploratory clinical trials. Curr Opin Oncol2012;24:338-344.CrossrefPubMed Google Scholar
9. Li SP , PadhaniAR. Tumor response assessments with diffusion and perfusion MRI. J Magn Reson Imaging2012;35:745-763.CrossrefPubMed Google Scholar
10. Oto A , FanX, MustafiD, et al.. Quantitative analysis of dynamic contrast enhanced MRI for assessment of bowel inflammation in Crohn’s disease pilot study. Acad Radiol2009;16:1223-1230. Google Scholar
11. Sourbron SP , BuckleyDL. Classic models for dynamic contrast-enhanced MRI. NMR Biomed2013;26:1004-1027.CrossrefPubMed Google Scholar
12. Gauthier L , DinhL, BeauléPE. Peri-acetabular bone mineral densityin total hip replacement. Bone Joint Res2013;2:140-148.CrossrefPubMed Google Scholar
13. Cron GO , SantyrG, KelczF. Accurate and rapid quantitative dynamic contrast-enhanced breast MR imaging using spoiled gradient-recalled echoes and bookend T(1) measurements. Magn Reson Med1999;42:746-753.CrossrefPubMed Google Scholar
14. Tofts PS . Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging1997;7:91-101.CrossrefPubMed Google Scholar
15. Parker GJ , RobertsC, MacdonaldA, et al.. Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn Reson Med2006;56:993-1000.CrossrefPubMed Google Scholar
16. Padhani AR , HayesC, LandauS, LeachMO. Reproducibility of quantitative dynamic MRI of normal human tissues. NMR Biomed2002;15:143-153.CrossrefPubMed Google Scholar
17. Buckley DL , KershawLE, StaniszGJ. Cellular-interstitial water exchange and its effect on the determination of contrast agent concentration in vivo: dynamic contrast-enhanced MRI of human internal obturator muscle. Magn Reson Med2008;60:1011-1019.CrossrefPubMed Google Scholar
18. Campbell PA , KungMS, HsuAR, JacobsJJ. Do retrieval analysis and blood metal measurements contribute to our understanding of adverse local tissue reactions?Clin Orthop Relat Res2014;472:3718-3727.CrossrefPubMed Google Scholar
19. Grammatopoulos G , PanditH, KamaliA, et al.. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J Bone Joint Surg [Am]2013;95-A:e81.CrossrefPubMed Google Scholar
20. Willert HG , BuchhornGH, FayyaziA, et al.. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg [Am]2005;87-A:28-36.CrossrefPubMed Google Scholar
21. Granchi D , CenniE, TrisolinoG, GiuntiA, BaldiniN. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater2006;77:257-264.CrossrefPubMed Google Scholar
22. Ingrisch M , SourbronS. Tracer-kinetic modeling of dynamic contrast-enhanced MRI and CT: a primer. J Pharmacokinet Pharmacodyn2013;40:281-300.CrossrefPubMed Google Scholar
23. Essig M , NguyenTB, ShiroishiMS, et al.. Perfusion MRI: the five most frequently asked clinical questions. AJR Am J Roentgenol2013;201:W495-510.CrossrefPubMed Google Scholar
24. Hargreaves BA , WortersPW, PaulyKB, et al.. Metal-induced artifacts in MRI. AJR Am J Roentgenol2011;197:547-555.CrossrefPubMed Google Scholar
25. Toms AP , MarshallTJ, CahirJ, et al.. MRI of early symptomatic metal-on-metal total hip arthroplasty: a retrospective review of radiological findings in 20 hips. Clin Radiol2008;63:49-58.CrossrefPubMed Google Scholar
26. Wynn-Jones H , MacnairR, WimhurstJ, et al.. Silent soft tissue pathology is common with a modern metal-on-metal hip arthroplasty. Acta Orthop2011;82:301-307.CrossrefPubMed Google Scholar
27. Anderson H , TomsAP, CahirJG, et al.. Grading the severity of soft tissue changes associated with metal-on-metal hip replacements: reliability of an MR grading system. Skeletal Radiol2011;40:303-307.CrossrefPubMed Google Scholar
28. Garbuz DS , HargreavesBA, DuncanCP, et al.. The John Charnley Award: diagnostic accuracy of MRI versus ultrasound for detecting pseudotumors in asymptomatic metal-on-metal THA. Clin Orthop Relat Res2014;472:417-423.CrossrefPubMed Google Scholar
29. Latteier MJ , BerendKR, LombardiAVJr, et al.. Gender is a significant factor for failure of metal-on-metal total hip arthroplasty. J Arthroplasty2011;26:19-23.CrossrefPubMed Google Scholar
30. Glyn-Jones S , PanditH, KwonYM, et al.. Risk factors for inflammatory pseudotumour formation following hip resurfacing. J Bone Joint Surg [Br]2009;91-B:1566-1574.CrossrefPubMed Google Scholar
31. Brinkley TE , Jerosch-HeroldM, FolsomAR, et al.. Pericardial fat and myocardial perfusion in asymptomatic adults from the Multi-Ethnic Study of Atherosclerosis. PLoS One2011;6:e28410.CrossrefPubMed Google Scholar
32. Kastrup A , LiT-Q, GloverGH, KrügerG, MoseleyME. Gender differences in cerebral blood flow and oxygenation response during focal physiologic neural activity. J Cereb Blood Flow Metab1999;19:1066-1071.CrossrefPubMed Google Scholar
33. Lohmann CH , MeyerH, NuechternJV, et al.. Periprosthetic tissue metal content but not serum metal content predicts the type of tissue response in failed small-diameter metal-on-metal total hip arthroplasties. J Bone Joint Surg [Am]2013;95-A:1561-1568. Google Scholar
34. Griffin WL , FehringTK, KudrnaJC, et al.. Are metal ion levels a useful trigger for surgical intervention?J Arthroplasty2012;27:32-36.CrossrefPubMed Google Scholar
35. Langton DJ , JoyceTJ, JamesonSS, et al.. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg [Br]2011;93-B:164-171.CrossrefPubMed Google Scholar
36. Axel L . Blood flow effects in magnetic resonance imaging. AJR Am J Roentgenol1984;143:1157-1166.PubMed Google Scholar









